
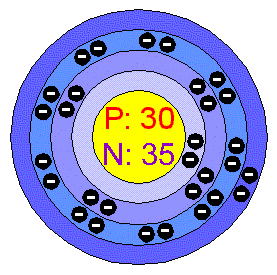
It is vital for the maintenance of bones and teeth.Ĭalcium ions on bone surfaces interact with those present in the bodily fluids, therefore enabling ion exchange, which is essential in maintaining the balance of calcium in the blood and bone.Ĭalcium circulating in the blood is involved in several vital processes including coagulation, nerve signal transmission, hormone signaling, and muscle contraction.Ĭalcium may be used as a reducing agent in the process of metal extraction.Ĭalcium is also used in the production of some metals, as an alloying agent.Ĭalcium carbonate is used to make cement and mortar and also in the glass industry. The biological use of calcium is to provide strength and structure to the skeleton. The atomic data of calcium is as follows: The crystal structure of calcium is cubic. So we can see it has 2 electrons in its valence shell, hence its valency is 2. 20 electrons occupy available electron shells (rings). The nuclear composition of an atom of calcium-40 (atomic number: 20), the most common isotope of this element consists of 20 protons and 20 neutrons. The density of calcium is 1.54 gm/cm³.Important isotopes of calcium include 48Ca, 46Ca, 44Ca, 43Ca, 42Ca, and 40Ca.CAS number for calcium is. It has a melting point of 842°c and a boiling point of 1484°c. At 20°c, this element is present in solid-state. The location of calcium elements in the modern periodic table is the 2nd group,4th period, and 's' block. The chemical symbol of calcium is 'Ca'.Its atomic number is 20 and its atomic mass is 40.078 g/mol. There are various uses of calcium and it is one of the most important chemical elements. In the physiological and biochemical processes of organisms and cells, calcium ions have an important role as electrolytes. Calcium is found to be the most abundant metal and when talking about the human body it is the fifth most abundant element in the human body. Sir Humphry Davy discovered calcium in 1808. Its physical and chemical properties are most similar to its heavier homologs strontium and barium. Calcium being an alkaline earth metal, it forms a dark oxide-nitrate layer due to its high reactivity and when exposed to the air. Group 2 elements of the modern periodic table are known as alkaline earth metals (except Beryllium).


 0 kommentar(er)
0 kommentar(er)
